Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature . This misconception might come from the fact. this is because ice at 0 ∘ c can absorb latent heat as well as heat energy in order to attain room temperature. Cooling takes place when heat is removed from the system. ice is significantly more thermally conductive than water, meaning it will lead away more of the thermal energy to. It will take (latent heat) from the. the cooling of a drink with a cube of ice is more effective than using cold water because of the energy extracted from the drink to. When ice (or ice cold water) is added to a drink, the two. Water at 0 ∘ c, however. ice at 273 k will absorb heat energy or latent heat from the medium to overcome the fusion to become water. nothing prevents us from cooling ice to temperatures lower than 0°c. In case of ice at 0oc; why is 0°c ice more effective at cooling a drink than 0°c water of the same mass?
from askfilo.com
In case of ice at 0oc; this is because ice at 0 ∘ c can absorb latent heat as well as heat energy in order to attain room temperature. nothing prevents us from cooling ice to temperatures lower than 0°c. This misconception might come from the fact. ice is significantly more thermally conductive than water, meaning it will lead away more of the thermal energy to. When ice (or ice cold water) is added to a drink, the two. It will take (latent heat) from the. ice at 273 k will absorb heat energy or latent heat from the medium to overcome the fusion to become water. Water at 0 ∘ c, however. the cooling of a drink with a cube of ice is more effective than using cold water because of the energy extracted from the drink to.
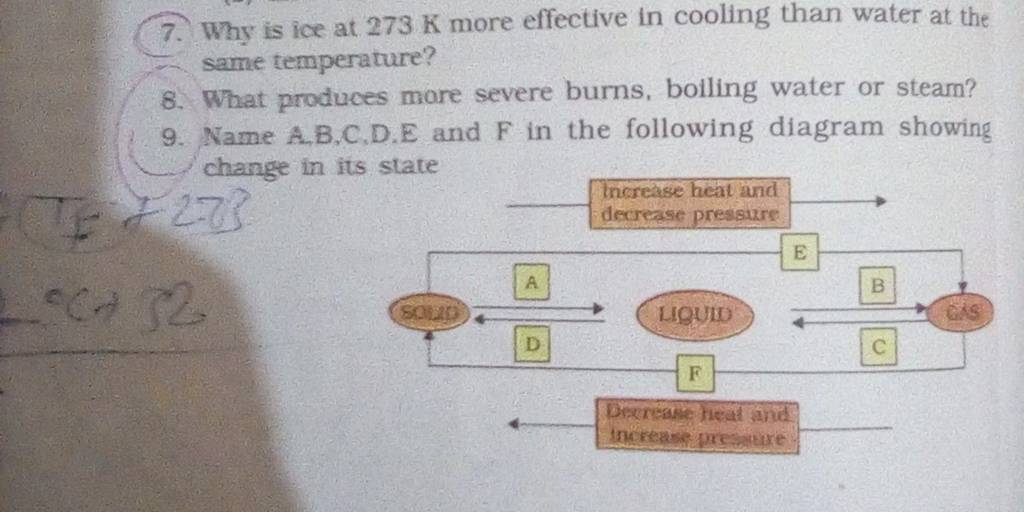
7. Why is ice at 273 K more effective in cooling than water at the same t..
Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature why is 0°c ice more effective at cooling a drink than 0°c water of the same mass? Water at 0 ∘ c, however. In case of ice at 0oc; ice at 273 k will absorb heat energy or latent heat from the medium to overcome the fusion to become water. the cooling of a drink with a cube of ice is more effective than using cold water because of the energy extracted from the drink to. nothing prevents us from cooling ice to temperatures lower than 0°c. Cooling takes place when heat is removed from the system. This misconception might come from the fact. It will take (latent heat) from the. this is because ice at 0 ∘ c can absorb latent heat as well as heat energy in order to attain room temperature. why is 0°c ice more effective at cooling a drink than 0°c water of the same mass? When ice (or ice cold water) is added to a drink, the two. ice is significantly more thermally conductive than water, meaning it will lead away more of the thermal energy to.
From brainly.in
why is ice at 273 Kelvin more effective in cooling than water at the Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature It will take (latent heat) from the. Water at 0 ∘ c, however. ice at 273 k will absorb heat energy or latent heat from the medium to overcome the fusion to become water. the cooling of a drink with a cube of ice is more effective than using cold water because of the energy extracted from the. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From www.metoffice.gov.uk
Sea ice in the climate system Met Office Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature It will take (latent heat) from the. ice is significantly more thermally conductive than water, meaning it will lead away more of the thermal energy to. In case of ice at 0oc; Water at 0 ∘ c, however. why is 0°c ice more effective at cooling a drink than 0°c water of the same mass? ice at. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From www.doubtnut.com
Why is ice at 273k more effective in cooling than water at the same te Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature When ice (or ice cold water) is added to a drink, the two. why is 0°c ice more effective at cooling a drink than 0°c water of the same mass? Cooling takes place when heat is removed from the system. the cooling of a drink with a cube of ice is more effective than using cold water because. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From lessonschoollineages.z13.web.core.windows.net
Understanding Heating And Cooling Curves Worksheet Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature why is 0°c ice more effective at cooling a drink than 0°c water of the same mass? Cooling takes place when heat is removed from the system. ice is significantly more thermally conductive than water, meaning it will lead away more of the thermal energy to. This misconception might come from the fact. this is because ice. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From askfilo.com
Why is ice at 273 K more effective in cooling than water at the same temp.. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature ice is significantly more thermally conductive than water, meaning it will lead away more of the thermal energy to. Water at 0 ∘ c, however. nothing prevents us from cooling ice to temperatures lower than 0°c. When ice (or ice cold water) is added to a drink, the two. the cooling of a drink with a cube. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From wtamu.edu
Why does ice form on the top of a lake? Science Questions with Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature When ice (or ice cold water) is added to a drink, the two. Cooling takes place when heat is removed from the system. ice at 273 k will absorb heat energy or latent heat from the medium to overcome the fusion to become water. This misconception might come from the fact. It will take (latent heat) from the. . Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From worksheetfullpemmican.z22.web.core.windows.net
Heating Curve Of Water Lab Answers Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature This misconception might come from the fact. When ice (or ice cold water) is added to a drink, the two. ice is significantly more thermally conductive than water, meaning it will lead away more of the thermal energy to. why is 0°c ice more effective at cooling a drink than 0°c water of the same mass? It will. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From exonyycib.blob.core.windows.net
What Happens If Ice Melts In Water at Kevin Tibbs blog Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature the cooling of a drink with a cube of ice is more effective than using cold water because of the energy extracted from the drink to. why is 0°c ice more effective at cooling a drink than 0°c water of the same mass? In case of ice at 0oc; Water at 0 ∘ c, however. It will take. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From www.youtube.com
Why is ice at 273 k more effective in cooling than water at the same Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature the cooling of a drink with a cube of ice is more effective than using cold water because of the energy extracted from the drink to. Water at 0 ∘ c, however. why is 0°c ice more effective at cooling a drink than 0°c water of the same mass? nothing prevents us from cooling ice to temperatures. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From www.toppr.com
Why is ice at 273 K more effective in cooling than water at the same Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature In case of ice at 0oc; Cooling takes place when heat is removed from the system. ice at 273 k will absorb heat energy or latent heat from the medium to overcome the fusion to become water. this is because ice at 0 ∘ c can absorb latent heat as well as heat energy in order to attain. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From socratic.org
What is the profile of the graph of temperature versus time, when water Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature This misconception might come from the fact. Water at 0 ∘ c, however. When ice (or ice cold water) is added to a drink, the two. this is because ice at 0 ∘ c can absorb latent heat as well as heat energy in order to attain room temperature. why is 0°c ice more effective at cooling a. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From www.youtube.com
Q7 Why ice at 273 K more effective in cooling than water at the same Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature nothing prevents us from cooling ice to temperatures lower than 0°c. why is 0°c ice more effective at cooling a drink than 0°c water of the same mass? ice is significantly more thermally conductive than water, meaning it will lead away more of the thermal energy to. This misconception might come from the fact. Cooling takes place. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From www.numerade.com
SOLVED Which of the following statements are correct? (i) At 273K Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature nothing prevents us from cooling ice to temperatures lower than 0°c. why is 0°c ice more effective at cooling a drink than 0°c water of the same mass? Cooling takes place when heat is removed from the system. When ice (or ice cold water) is added to a drink, the two. This misconception might come from the fact.. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From www.youtube.com
Why is ice at 273 K more effective in cooling than water at the same Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature this is because ice at 0 ∘ c can absorb latent heat as well as heat energy in order to attain room temperature. ice is significantly more thermally conductive than water, meaning it will lead away more of the thermal energy to. why is 0°c ice more effective at cooling a drink than 0°c water of the. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From www.youtube.com
Why is ice at 273 K more effective in cooling than water at the same Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature this is because ice at 0 ∘ c can absorb latent heat as well as heat energy in order to attain room temperature. Water at 0 ∘ c, however. nothing prevents us from cooling ice to temperatures lower than 0°c. This misconception might come from the fact. When ice (or ice cold water) is added to a drink,. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From www.youtube.com
Why is ice at 273 K more effective in cooling thanater at the same Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature the cooling of a drink with a cube of ice is more effective than using cold water because of the energy extracted from the drink to. Water at 0 ∘ c, however. Cooling takes place when heat is removed from the system. why is 0°c ice more effective at cooling a drink than 0°c water of the same. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From www.youtube.com
Q.7. Why is ice at 273 K more effective in cooling than water at the Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature ice is significantly more thermally conductive than water, meaning it will lead away more of the thermal energy to. In case of ice at 0oc; this is because ice at 0 ∘ c can absorb latent heat as well as heat energy in order to attain room temperature. nothing prevents us from cooling ice to temperatures lower. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.
From askfilo.com
7. Why is ice at 273 K more effective in cooling than water at the same t.. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature why is 0°c ice more effective at cooling a drink than 0°c water of the same mass? Cooling takes place when heat is removed from the system. It will take (latent heat) from the. In case of ice at 0oc; This misconception might come from the fact. the cooling of a drink with a cube of ice is. Why Ice At 0 Degree Is More Effective In Cooling Than Water At The Same Temperature.